
Gideon Fraenkel
Emeritus Professor
1136 Newman & Wolfrom Laboratory
100 W 18th Ave
Columbus, OH 43210
Areas of Expertise
- Organic Chemistry
Bio
Gideon Fraenkel, M. S. Newman Professor of Chemistry, received his B.S. degree from the University of Illinois-Urbana in 1952, his M.S. from Harvard in1953, and his Ph.D. from Harvard in 1957; He was a Research Fellow at the California Institute of Technology, 1957-1960; assistant professor at the Ohio State University, 1960-1964; associate professor, 1965-1967; and full professor 1967 to the present. He was a guest professor at the University of Lund in 1971, at the University of Göthenberg in 1990 and at the University of Bari in 2007. His honors and awards include: Noyes Fellow, California Institute of Technology, 1958; NSF Award for Creativity, 1975 and 1990; Fellow of the American Association for the Advancement of Science, 1996; and M. S. Newman Professor of Chemistry, 1999.
Professor Fraenkel's research is in the general area of physical organic chemistry, organometallic chemistry, and NMR and has been supported continuously by mainly NSF, as well as NIH and AFOSR. His research is described in over 200 articles and a book, "NMR of Chemically Exchanging Systems", which describes Professor Fraenkel's pioneering contributions to the theory and applications of dynamic NMR. He is a consultant with Monsanto, 1996 to the present; Firestone Tire and Rubber (now Bridgestone), 1978-1983; Goodyear, 1984 to the present; and Jones, Day, Reavis and Pogue, 1999 to the present.
Research Overview
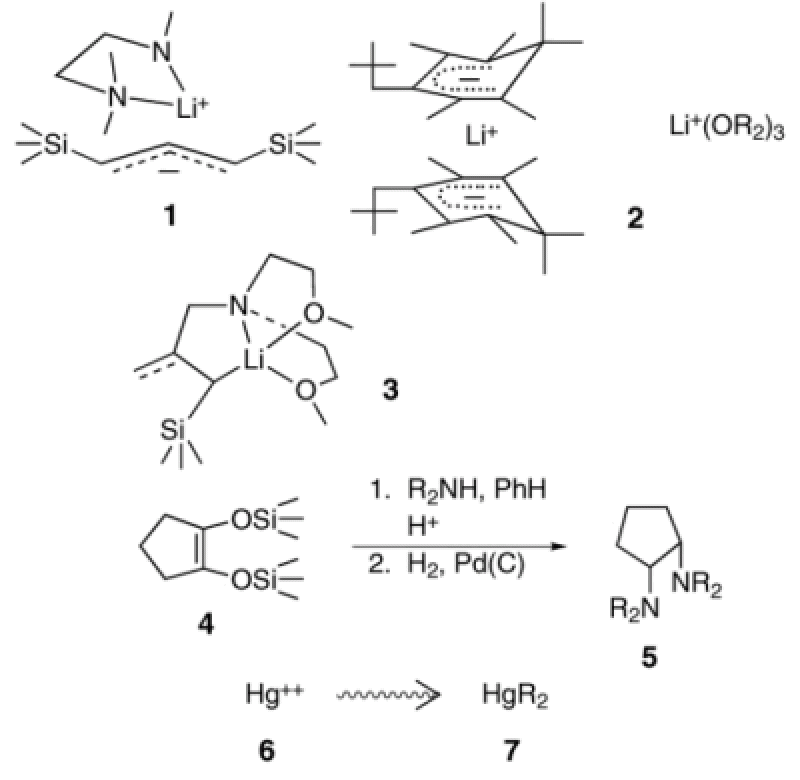
Our research interests revolve around carbanionic substances, their structures and dynamic behavior. For example, we prepare potentially theoretically interesting and synthetically useful organolithium compounds enriched in 13C and 6Li. By exploiting 13C-6Li, spin-coupling, one can determine the structures of the (RLi)n clusters, Having establlished the density matrix theory for NMR of rapidly reorganizing chemical systems, we have applied it to study the kinetics and mechanisms of fast-equilibrium reorganization processes such as inversion at carbanionic carbon, C-Li bond exchange and interconversion of species. These latter techniques have also been used to determine the structure (see 1 and 2) of ion-paired salts for the first time and to resolve the dynamics of their different fast equilibrium reorganizing processes. Typical ΔH‡ values for ion-ion reorientation in contact ion-pairs lie in the range 6 to 7 kcal/mol, respectively. In particular, lately, we have been able to produce a series of allyllithium compounds, which exhibit unique and varying degrees of delocalization and C-Li covalence as determined from our NMR and X-ray crystallographic studies. These otherwise aberrant structures (see 3) are stabilized by the use of ligands attached to the carbanionic moiety. If the ligand is too short to place Li+ normal to the center of the allyl plane (as in delocalized externally solvated allylic lithium compounds) lithium is sited off this vertical axis and some degree of C-Li covalence is established. Theoretical and structures are under investigation. Offshoots of our carbanion research include a new preparation of cis-cyclicdiamines (see 4->5) as potential ligands for metal ions, the use of nitrous oxide as a diazo transfer reagent and the physiological alkylation of toxic heavy metal ions (see 6->7). This unusual combination of skills from organic and organometallic synthesis, NMR theory and technology and calculations prepare members of the group to make many contributions in industry and academia. For example, given that aggregation and solvation correlate so closely with mode of reactivity it is now common practice in industry to monitor organolithium reagents prior to their use to ensure the reagents are in suitable form for their intended use. The group has also been very active worldwide in collaborations on problems involving dynamic NMR.
Recent Publications
Gideon Fraenkel, “Dynamics and Reorganization Behavior of Organolithium Compounds” in “The Chemistry of Organolithium Compounds” Rappoport, Z; Marek, I, Eds.; John Wiley: Chichester, UK, 2005, Vol. 2, Chap 1, pp. 1-67.
Fraenkel, G.; Chow, A.; Fleischer, R.; Liu, H. “Perturbation of Conjugation in Allylic Lithium Compounds due to Stereochemical Controll of Internal Coordination of Lithium: Crystallography, NMR and Calculational Studies, J. Am. Chem. Soc. 2005, 126, 14995.
Fraenkel, G.; Chen, X.; Chow, A.; Gallucci, J. C.; Liu, H. “Internally Solvated Cyclopentadienyl Lithium Compounds: Structural Integrity of the Cp-Li+ Moiety. NMR, Dynamics, X-ray Crystallography and Calculations” J. Org. Chem. 2005, 70, 9131.
Fraenkel, G.; Gallucci, J. C.; Liu, H. “Perturbation of Conjugation in Internally Solvated Allylic Lithium Compounds: Variation of Ligand Structure, NMR and X-ray Crystallography” J. Am. Chem. Soc. 2006, 128, 8211.
Fraenkel, G.; Chen, X.; Gallucci J. C.; Lu, Y. “Structural Distortions and Dynamic Behavior of the Elusive “L” Shaped cis-Bicyclo[3.3.0]octenyl-
Lithium: X-ray Crystallography and NMR Studies” J. Org. Chem. 2007, 72, 4961-4965.
Rieth, S.; Yan, Z.; Xia, S.; Gardlik, M.; Chow, A.; Fraenkel, G.; Hadad, C. M.; Badjic J. “Molecular Encapsulation via Metal-to-Ligand in a Cu(1)-Folded Molecular Basket” J. Org. Chem. 2008, 130, 5100-5108.
Fraenkel, G.; Chen, X.; Gallucci, J. “Arylallyllithium Compounds: Internally versus Externally Coordinated, Comparison of their Structures and Dynamic Behavior via X-ray Crystallography and NMR” J. Am. Chem. Soc. 2008, 130, 4140-4145.
Fraenkel, G.; Cabral, J.; Chen, X.; Ren, Y. “Comparison of Internally Coordinated 2-pentenyllithiun with its 4-Sila Analog. Structure and Dynamic Behavior. Unexpected C-13, Li-7 Spin Coupling, J. Org Chem. 2009, 74, 2311-2320.
